Perovskite Devices
Solid-state perovskite solar cell (PSC) was first reported in 2012, since then, power
conversion efficiency (PCE) has reached up to 25.8% (2022, NREL)
We investigate and develop highly efficient and stable perovskite solar cells and related
opto/electronic devices, mostly based on Atomic Layer Deposition (ALD)
Device structure of the PSCs contains 3 main layers. Perovskite, as the absorber layer and 2 charge transport layers; i.e, electron transport layer (ETL) and hole transport layer (HTL), respectively. Here at EML, we study the perovskite with various chemical compositions (MAPbI3, FAPbI3, and mixed cations) and its structural evolution using different characterization techniques: transmission electron microscope (TEM), X-ray diffraction (XRD), and scanning electron microscope (SEM). Electronic and optical properties of the perovskite are also studied at EML, along with studies of energy band structure, excitons behaviors, lattice vibrations, and charge transportation. From fundamental research such as crystallization kinetics of the FAPbI3 and strain engineering, to device physics such as interface-engineering and defect passivation, enabled us to achieve high PCE of 23.91% (Adv. Energy Mater. 2021)

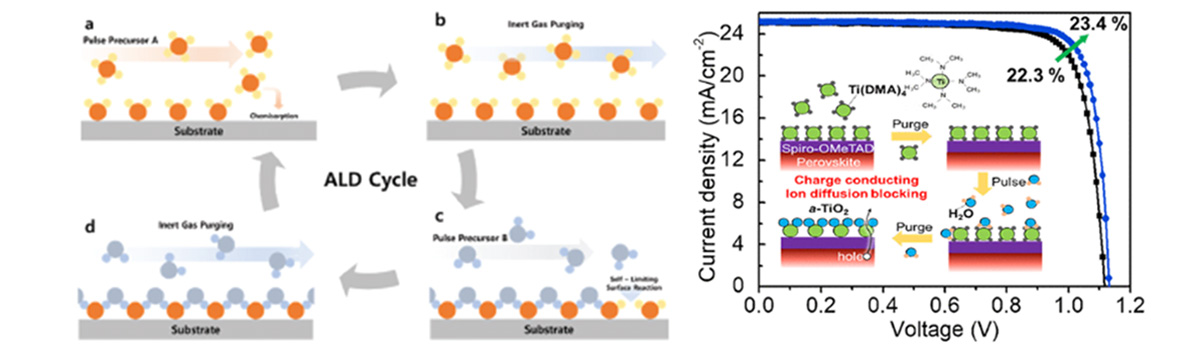
Using atomic layer deposition (ALD), we are able to deposit various metal oxides for the charge transport layers (TiO2, SnO2, ZnO, and NiO …etc.). With the advantage of precise thickness control and excellent coverage, highly compact layer of metal oxides can be deposited for charge transport. ALD process can be done even in low temperatures, which allows interface-engineering without damaging the layers. As a result, we have improved the operational stability of the PSCs by coating the amorphous TiO2 on the HTL (ACS Energy Lett. 2021)
Material Analysis
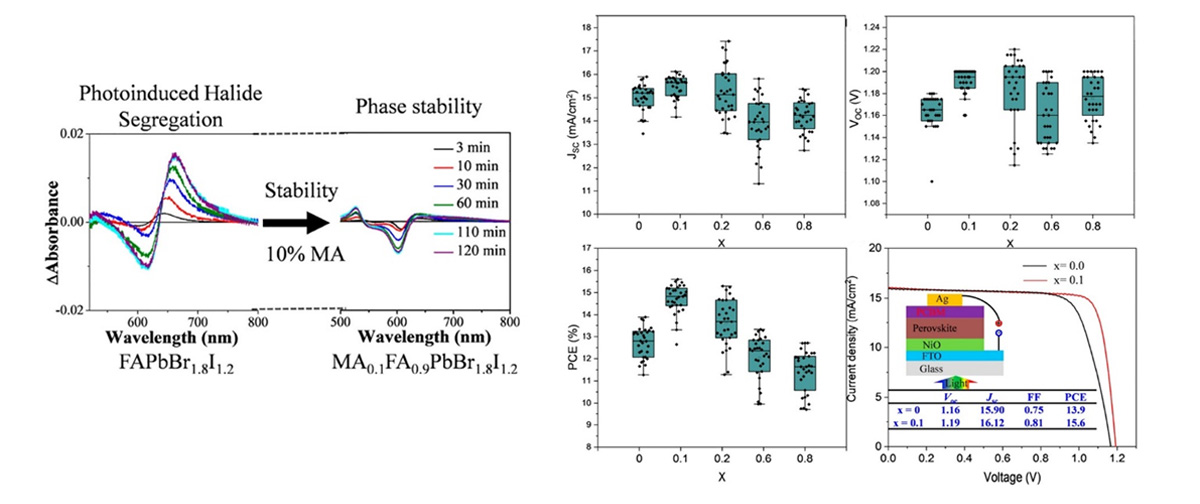
Mixed halide perovskites as top absorbers with relatively high bandgaps (Eg > 1.70 eV) are essential for tandem and multijunction solar cells. Light-induced phase segregation of mixed halide perovskites limits their commercialization, and it has been found that organic cation substitution in the A site can suppress photoinduced phase segregation. By substituting methylammonium (MA+) ions into formamidinium (FA)PbBr1.8I1.2 perovskites, we probed photoinduced segregation at different temperatures. In this study, we found that the segregation rate constant increased with MA+ content; however, significant suppression of the rate constant was observed in the case of 10% substitution. The activation energy for photoinduced phase segregation increased (by ∼5 kJ mol–1) upon introduction of 10% MA+ into FAPbBr1.8I1.2, reflecting an increased energetic barrier for halide segregation. We also find the effect of A-site cation alloying against photoinduced phase segregation in active devices such as phototransistors and photovoltaic cells.